Tin(IV) chloride pentahydrate, lumps
IMPORTANT: Buying with a valid VAT ID?
Please contact us by email: info@widerangemetals.com in order to proceed your order using the zero (0%) VAT rate.
For our clients, we are offering posibilyti to achieve the best results using high quality materials. You have the opportunity to buy a quality product with a wide range of uses, we believe that this product will meet all your requirements.
Tin(IV) chloride pentahydrate is a tin source compatible with chlorides. Biologically, it induces a slight inhibition in the catalytic activity of the enzyme horse liver alcohol dehydrogenase (HLADH). The pentahydrate is substitute for its anhydrous grade in applications where presence of water is permissible. Historically, stannous chloride was used as warfare chemical as it forms thick HCl fumes when in contact with water.
CAS no. 10026-06-9
EINECS no. 600-048-8
Molecular formula SnCl4.5H2O
Molecular weight 350.6
Structure: 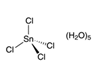
Manufacture:
Stannic chloride is produced by chlorination of metallic tin by chlorine gas.
Synonyms:
Stannic chloride pentahydrate [UN2440] [Corrosive]
Tin(IV) chloride pentahydrate
Tetrachlorostannane pentahydrate
MFCD00149864
tin(iv) tetrachloride pentahydrate
EINECS 231-588-9
Stannane, tetrachloro-, hydrate (1:5)
tetrachlorostannane, pentahydrate
Applications:
Stannic chloride pentahydrate has various end uses:
In production of Xyrogel that is applied in solid state gas censors
In preparation of indium tin oxide thin films for semiconductor industry
As mordant in fabric dyeing
As oxidant of acid dyes and in post-treatment of aniline dyes
It gives organo tin compounds (SnR4) when treated with Grignards reagent
As catalyst in acylation of thiophene to 2-acetylthiophene
As reactant in manufacture of APIs and agrochemicals
As catalyst in production of polystyrene
As highly efficient disinfectant
In conductive coatings, polymers, resins, and wood preservatives
Stability: Stable, but may decompose in contact with moisture or water. Incompatible with strong acids.
Water solubility: Soluble
You are customer from Europe Union? And buying with a valid VAT ID?
Please contact us by email: info@widerangemetals.com in order to proceed your order using the zero (0%) VAT rate.
What You Need to Know About Stannic Chloride
The name stannic chloride is relatively unknown to many people, especially compared to other inorganic compounds. Most people have the gist when they hear the word “chloride.” However, it only shows the components and not the usage.
The compound is also known as tin iv chloride pentahydrate. So you may have heard or known it by its other name. To be sure, you can double-check with the molecular formula and CAS registry. Numerous items can look or even sound alike at a glance, but it requires the user’s due diligence and understanding of what they have in hand. There’s nothing wrong with checking multiple times, ensuring the correct chemical compound and grade.
What is Stannic Chloride?
This compound is colorless with a pungent odor. Some of the most common forms are liquid, but it’s also common in solid form. In solid form, the name changes to stannic chloride pentahydrate to show that it needs rehydration before use. At any rate, it’s a hazardous chemical that is dangerous upon ingestion and inhalation.
Since it’s water soluble, stannic chloride commonly leaves the laboratory already as a solution or part of other compounds. One of the most common is in the semiconductor manufacturer. Stannic chloride also has good conductive properties.
But one of the usages that many people will notice is that stannic chloride took part in warfare as a chemical weapon. As it is water-soluble, putting stannic chloride in water will create a thick poisonous gas that won’t leave a trace.
Structure tin iv chloride pentahydrate
The molecular structure of tin iv chloride pentahydrate is SnCl4.5H2O. Many people often confuse stannous chloride with stannic chloride. Both have similar formulas and names. However, Stannic Chloride has four oxidation states of tine, while stannous only have two.
To ensure that you choose the right chemical compound, you can look at the CAS registry number, as each compound has a different register. For the tin iv chloride pentahydrate formula, the CAS number is 10026-06-9.
The compound results from heating chlorine gas and tin at 115 degrees Celsius or 239 Fahrenheit. Stannic Chloride is common as liquid, but freezing at 33 celsius will solidify the compound. Freezing the compound will give the chloride isostructural SnBr4. Another common form is the pentahydrate form. Being in the hydrate form gives the structure of the additional water molecule that connects the other molecules through hydrogen.
Organic synthesis
The consensus on the organic and inorganic compounds is on the existence of the Carbon atom. The organic compound has carbon, and the inorganic one doesn’t. But even being an inorganic compound, stannic chloride can help organic synthesis.
The most common use is in Friedel-Craft reactions. The conductive nature makes stannic chloride another popular choice for the acetylation process. As one of the well-known Lewis acid catalysts, SnCl4 is highly useful to acetylate thiophene. Most laboratories choose aluminum chloride as their catalyst, but many choose stannic chloride when they aim to include the nitration process.
Chloride pentahydrate industrial Use
The most common usage for stannic chloride pentahydrate is in the conductor manufacturer industries. Numerous plating industries use this chloride in their manufacturing process. The compound is the reaction of tin metal and chlorine gas at 115 degrees Celsius. Therefore, it has good conductive nature.
Another industry that uses stannic chloride is the catalyst and polymer stabilizers. Massive industries in nanoplate and preservatives often use stannic chloride even more so when they have SnO2 coat preparation in the process.
On the other hand, stannous chloride is common in the silk and textile industry. Numerous companies are using this compound to weigh the dye on textile production. It also acts as a mordant and stabilizer for the dye. Therefore, the color will stick longer and better.
The industry also often sees cadmium chloride hemi pentahydrate as one of the compounds they process with stannic chloride. This additional chemical acts as a compound that conducts electricity well. Therefore, it’s common to see this combination in the automotive industry and electronic appliance manufacturers.
Research Use
Historically, the compound is one of the ingredients vital to making the atomic bomb. And since it’s water soluble, it possesses even more risks for the public. Therefore, any company manufacturing this chemical strictly limits sales to legit organizations and universities. Anyone who is looking to buy will have to input their affiliation info and have the company verify it. Not to mention how easy it is to mistake the compound for other chemical compounds such as stannous chloride.
For the time being, physics and chemical labs are exploring the compound to find new usage. They are looking for a usage that is both scalable and benefits the public at the same time. Some of the current uses are thanks to the relentless research in the past.
Stannic chloride pentahydrate other uses
Based on the history, the compound is not strictly for industrial and research purposes only. Any organization or laboratory looking for stannic chloride pentahydrate buy can arrange with the third-party vendor or directly with the manufacturer to obtain this compound. They will require the organization to submit proof and ensure its legitimacy.
The law on chemical sales is restrictive as the product can’t transport like regular products due to the hazard warnings. The necessary knowledge of handling during and after transportation is also crucial for everyone involved.
Safety and sales of tin chloride pentahydrate
Due to its hazardous nature, you should handle stannic chloride pentahydrate with extra care. Use the necessary safety unit to prevent any accidents. There are some companies or websites offering tin chloride pentahydrate cheap. If that’s the case, you need to inquire for more information. Remember that there are several grades of chemical compounds. Make sure that you choose the quality that fit your purposes. And that goes the same for the cadmium chloride hemi pentahydrate.
Most companies offer competitive prices, but at the cost of the safety and grade of the customers. It is the customer’s discretion to understand what they ordered and need, given they already have enough knowledge on how to handle hazardous chemicals like stannic chloride.



